Abstract
Neurodegenerative disorders are the result of cumulative damages to parts of the brain over time. Ethanol-induced cerebellar dysfunction, ataxia, Wernicke encephalopathy in alcoholics, exposure to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in MPTP-induced Parkinsonism, post-infection Guillain-Barré, encephalitis lethargica and possibly Parkinson’s disease (PD), are all examples of targeted destruction of neurons predetermined by substances that bind selectively to specific receptors on neurons. Why MPTP selectively knocks out much of the pars compacta of the substania nigra? why alcohol abuse affects the cerebellum more than other regions? Why viral infections such as the seasonal flu or SARS-CoV-2 affect the olfactory bulb and the sense of smell ? These questions ought to make you think about a common denominator ” receptor selectivity and targeted destruction by pathogens”. Here, a brief review of past and current literature is provided in an attempt to answer the above questions, and to discuss the role of an important receptor implicated in many neurological disorders with possible infectious origins, called the angiotensin converting enzyme 2 (ACE2). Although easier said than done, This receptor-mediated target-specific destructive force creates an amazing possibility to predict neuropathology and deliver targeted genetic changes to neurons in specific regions of the brain using the properties of tissue tropism .
Table of Contents
- History and Background: Encephalitis lethargica and MPTP-induced Parkinsonism
- Renin Angiotensin system and ACE2
- RAS and ACE2 in the Brain
- ACE2 Receptors and the SARS Coronavirus outbreak of 2003 and the Novel SARS-CoV-2 (COVID-19)
- Viral Infections and Autoimmune Disorders: Predictive value of Non-specific Symptoms such as Anosmia
- Putting the Pieces Together
- References
History and Background: Encephalitis lethargica and MPTP-induced Parkinsonism
The movie “Awakening” starring Robin Williams and Robert De Niro is a film made based on the late Oliver Sacks’ memoir with the same title describing his encounters with patients with encephalitis lethargica (EL) or sleeping sickness in the early 1970’s (Allan, 2007; Dale et al., 2004; Sacks, 1983). In 1916, von Economo first described EL, as a CNS disorder presenting with pharyngitis followed by sleep disorder, basal ganglia signs (particularly Parkinsonism) and neuropsychiatric sequelae (Dale et al., 2004). Since the 1916-1927 epidemic, only sporadic cases have been described of Pathological studies revealed an encephalitis of the midbrain and basal ganglia, with lymphocyte (predominantly plasma cell) infiltration. As a result of this epidemic (according to today’s definition by WHO, EL could be considered a pandemic) chronic hospitals were established in order to look after EL patients decades after (Dale et al., 2004). This is a case that provides evidence for tissue tropism; an ability of host cells or tissue to harbour pathogenic invaders. An interesting fact about this disease, although correlational, is that the Spanish flu pandemic also overlapped with the emergence of EL around the world. Raising suspicion in the infectious origin of EL and some neurological diseases. EL patients responded to L-dopa but after developing tolerance they fell back into catatonia and frozen state, despite being cognitively un-impaired (Sacks, 1983).
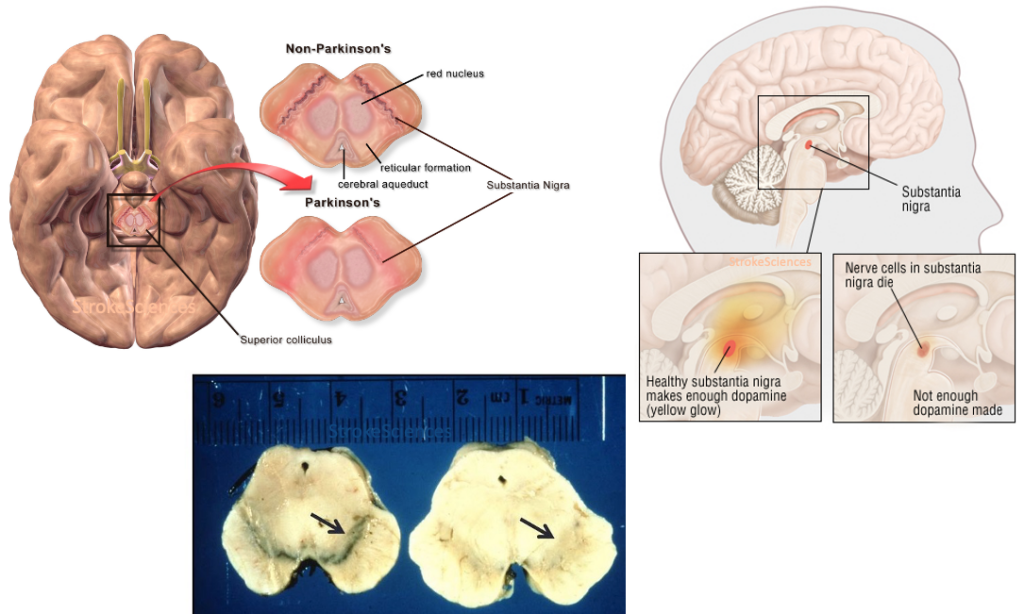
As he recounts in his almost forgotten book “The Case of the Frozen Addicts” published in 1984, Dr. William Langston was the Director of Neurology at the Santa Clara Valley Medical Center in San Jose, California when he encountered a surge of patients coming in with psychiatric and severe parkinsonian symptoms. Later it was discovered that an amateur chemist was making synthetic heroin in his apartment and either due to sloppy work or deliberately, traces of MPTP infiltrated in his final product causing a group of young adults in northern California to present with extreme Parkinsonism after using the drug. These patients also responded to L-dopa, but the effects wore off within months (Langston, 2017). Langston’s discovery led to greater insight into the selective damages to the substantia nigra pars compacta in Parkinson’s disease (PD) , the price of MPTP for research purposes shot up by almost 800%, and the coinage of the term “designer drug” (Snow et al., 2000). It is important to note that the first well-known study on the environmental factors contributing to the prevalence of PD was also published in 1986, where Barbeau and colleagues studied a homogenous population in Quebec, Canada, and found high rates of prevalence in areas of pesticide use, confirming the role of MPTP in PD (Barbeau et al., 1987).

Moving on to our dear companion in good and bad times. Throughout history, alcohol consumption has sparked heated debates amongst researchers and physicians. It is one of the main causes of trauma-related morbidity and mortality, organ damage and cardiovascular pathologies. Yet we consume it almost in any possible occasion; celebration of life, birthdays, promotions, demotions, getting sacked, breakups and so on. One could only imagine that it also could be a causative agent in procreation and/or divorce. Joking aside, alcohol is quite selective in where its metabolites land and dock and cause oxidative stress and mitochondrial damage in the brain and other organs (Andersen, 2004. Both acute and chronic alcohol exposure can increase production of reactive oxygen species and enhance peroxidation of lipids, protein, and DNA, as has been demonstrated in a variety of systems, cells, and species, including humans. Although the exact mechanism is not well-explored, alcoholic essential tremor is one of the pronounced features in chronic users and it is linked to cerebellar and brainstem damage (Andersen, 2004; Sullivan et al., 1995).
In the following sections an attempt is made to answer a few questions: Why certain substances selectively affect only certain regions in the brain? how do they cross the blood-brain-barrier, and how do they bind to specific receptors? The pharmaceutical industry has already taken action to decode such mechanisms in order to develop target-specific drug delivery to the brain (Dong, 2018). Such endeavours have paved the way for some recent explanatory models which will be discussed as follows.
Renin Angiotensin system and ACE2
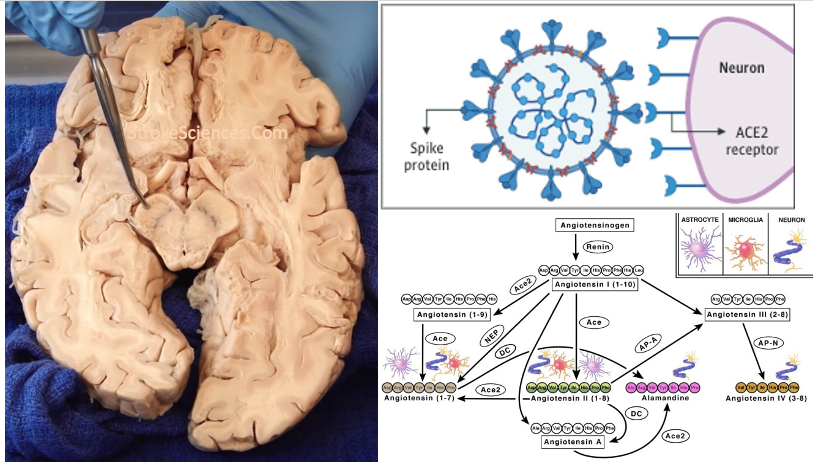
Renin angiotensin system (RAS) is an endocrine system widely known for its physiological roles in electrolyte homeostasis, body fluid volume regulation and cardiovascular control in peripheral circulation (Jackson et al., 2018). However, brain RAS is an independent form of RAS expressed locally in the brain, which is known to be involved in brain functions and disorders (Vaduganathan et al., 2020; Xia & Lazartigues, 2008). There is strong evidence for a major involvement of excessive brain angiotensin converting enzyme (ACE)/Angiotensin II (Ang II)/Angiotensin type-1 receptor (AT-1R) axis in increased activation of oxidative stress, apoptosis and neuroinflammation causing neurodegeneration in several brain disorders. Numerous studies have demonstrated strong neuroprotective effects by blocking AT1R in these brain disorders (Lenkei et al., 1997). Additionally, the angiotensin converting enzyme 2 (ACE2)/Angiotensin (1–7)/Mas receptor (MASR), is another axis of brain RAS which counteracts the damaging effects of ACE/Ang II/AT1R axis on neurons in the brain. Thus, angiotensin II receptor blockers (ARBs) and activation of ACE2/Angiotensin (1–7)/MASR axis may serve as an exciting and novel method for neuroprotection in several neurodegenerative diseases (Lenkei et al., 1997).
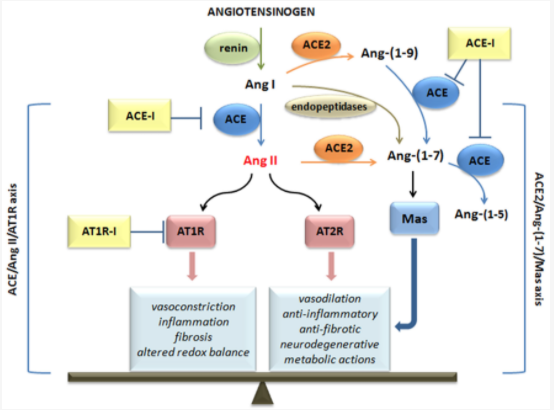
 effects mediated. (credit: D’Ardes et al., 2020)
effects mediated. (credit: D’Ardes et al., 2020)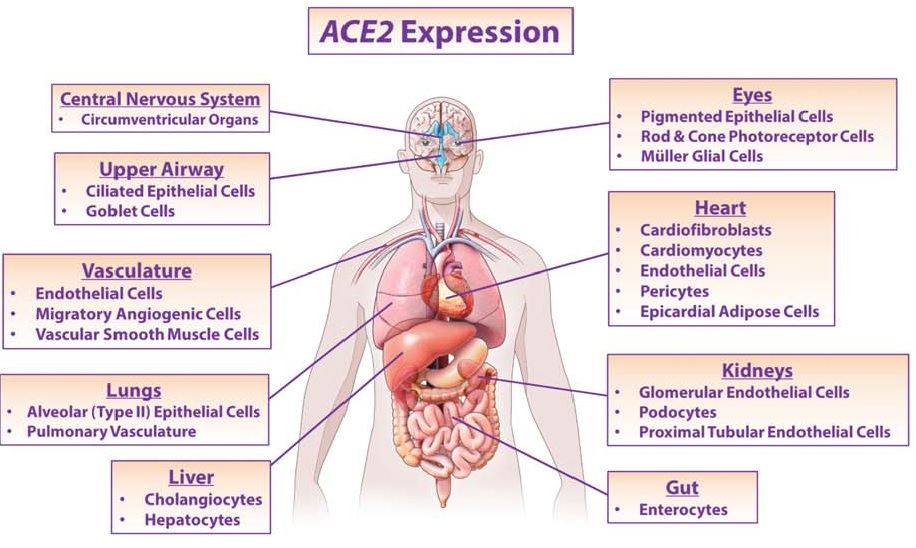
Angiotensin (Ang)‐converting enzyme (ACE) 2 cleaves Ang‐II into the vasodilator peptide Ang‐(1–7), thus acting as a pivotal element in balancing the local effects of these peptides. ACE2 has been identified in various tissues and is supposed to be a modulator of cardiovascular function. Decreases in ACE2 expression and activity have been reported in models of hypertension, heart failure, atherosclerosis, diabetic nephropathy and others. In addition, the expression level and/or activity are affected by other renin–angiotensin system components (e.g., ACE and AT1 receptors). Local inhibition or global deletion of brain ACE2 induces a reduction in baroreflex sensitivity (Hamming et al., 2004).
RAS and ACE2 in the Brain
For many years, modulators of the renin angiotensin system (RAS) have been trusted by clinicians for the control of essential hypertension (Jackson et al., 2018; Wright & Harding, 2013). It was recently demonstrated that these modulators have other pleiotropic properties independent of their hypotensive effects, such as enhancement of cognition. Within the brain, different components of the RAS have been extensively studied in the context of neuroprotection and cognition (Lenkei et al., 1997). Interestingly, a crosstalk between the RAS and other systems such as cholinergic, dopaminergic and adrenergic systems have been demonstrated (Wright & Harding, 2013.
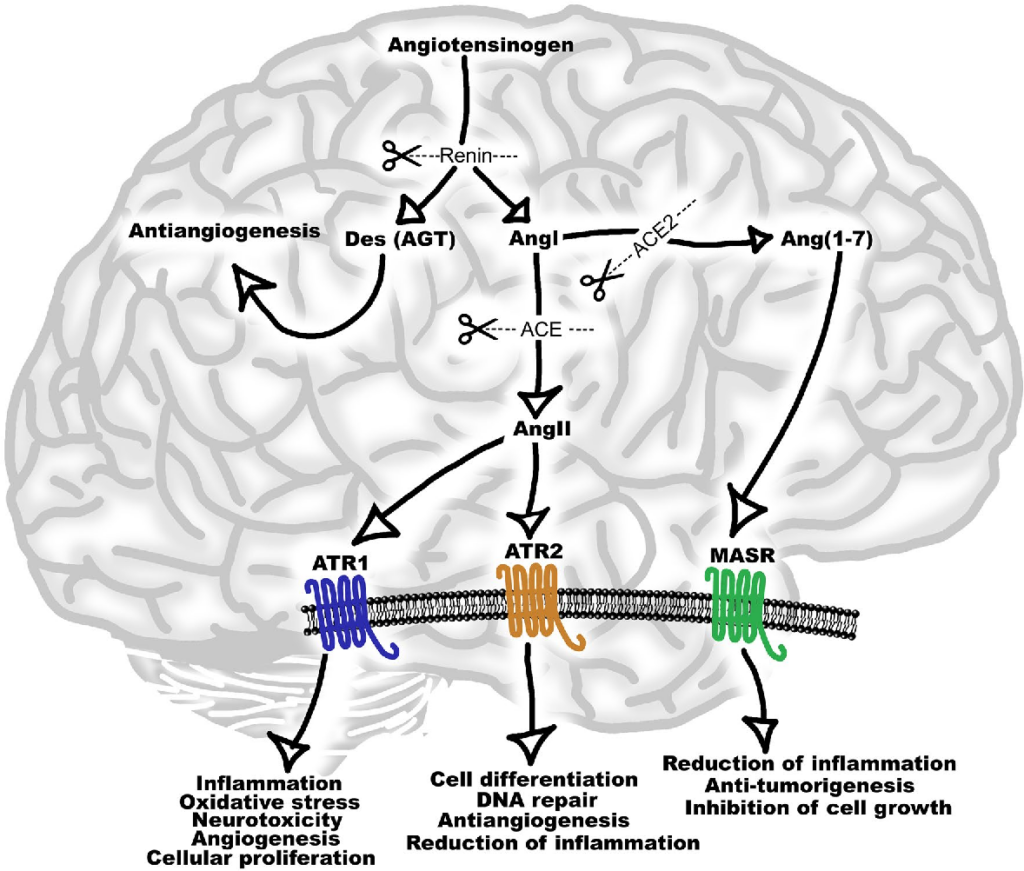
Within the brain, there are peripheral and central angiotensinergic pathways. The main peripheral pathway is the forebrain pathway which integrates the circumventricular organs (CVOs) that surround the third and fourth ventricles and consists of fenestrated capillaries to allow for the peripheral access of RAS components (Lenkei et al., 1997; Wright & Harding, 2013). Although structures along the forebrain pathway have access to peripheral RAS components, the majority of brain regions do not. Unlike local RAS production in other organs, the blood–brain barrier (BBB) restricts peripheral RAS components from accessing the majority of brain regions, making the local synthesis of cerebral RAS essential. The main central angiotensinergic pathway connects the hypothalamus and medulla and is the primary contributor of locally synthesized angiotensin (Vlot et al., 2017). To supplement this, RAS components are also synthesized in various other brain regions. Both peripheral and central RAS production contributes significantly to cardiovascular homeostasis. AT1R is classically known for its presence on endothelial cells and its role in vasoconstriction, and AT2R for its role in vasodilation and can be further reviewed here (Grammatopoulos et al., 2007).
ACE2 Receptors and the SARS Coronavirus outbreak of 2003 and the Novel SARS-CoV-2
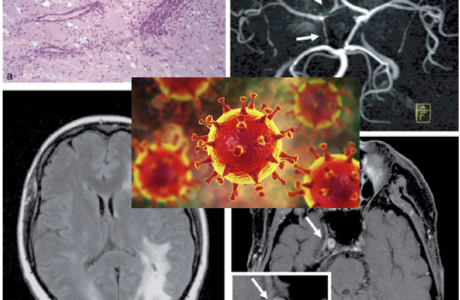
In 2004 Hamming and colleagues published a paper addressing the involvement of ACE2 and respiratory infections during the SARS outbreak. Severe acute respiratory syndrome (SARS) is an acute infectious disease that spreads mainly via the respiratory route. A distinct coronavirus (SARS-CoV) has been identified as the etiological agent of SARS. Recently, a metallopeptidase named angiotensin-converting enzyme 2 (ACE2) has been identified as the functional receptor for SARS-CoV (Chen et al., 2020; D’Ardes et al., 2020; Mao et al., 2020; Zubair et al., 2020). Although ACE2 mRNA is known to be present in virtually all organs, its protein expression is largely unknown (Hamming et al., 2004). Since identifying the possible route of infection has major implications for understanding the pathogenesis and future treatment strategies for SARS, the present study investigated the localization of ACE2 protein in various human organs (oral and nasal mucosa, nasopharynx, lung, stomach, small intestine, colon, skin, lymph nodes, thymus, bone marrow, spleen, liver, kidney, and brain) (Chen et al., 2020). The most remarkable finding was the surface expression of ACE2 protein on lung alveolar epithelial cells and enterocytes of the small intestine. Furthermore, ACE2 was present in arterial and venous endothelial cells and arterial smooth muscle cells in all organs studied (Chen et al., 2020). In conclusion, ACE2 is abundantly present in humans in the epithelia of the lung and small intestine, which might provide possible routes of entry for the SARS-CoV. This epithelial expression, together with the presence of ACE2 in vascular endothelium, also provides a first step in understanding the pathogenesis of the main SARS disease manifestations (Hamming et al., 2004).
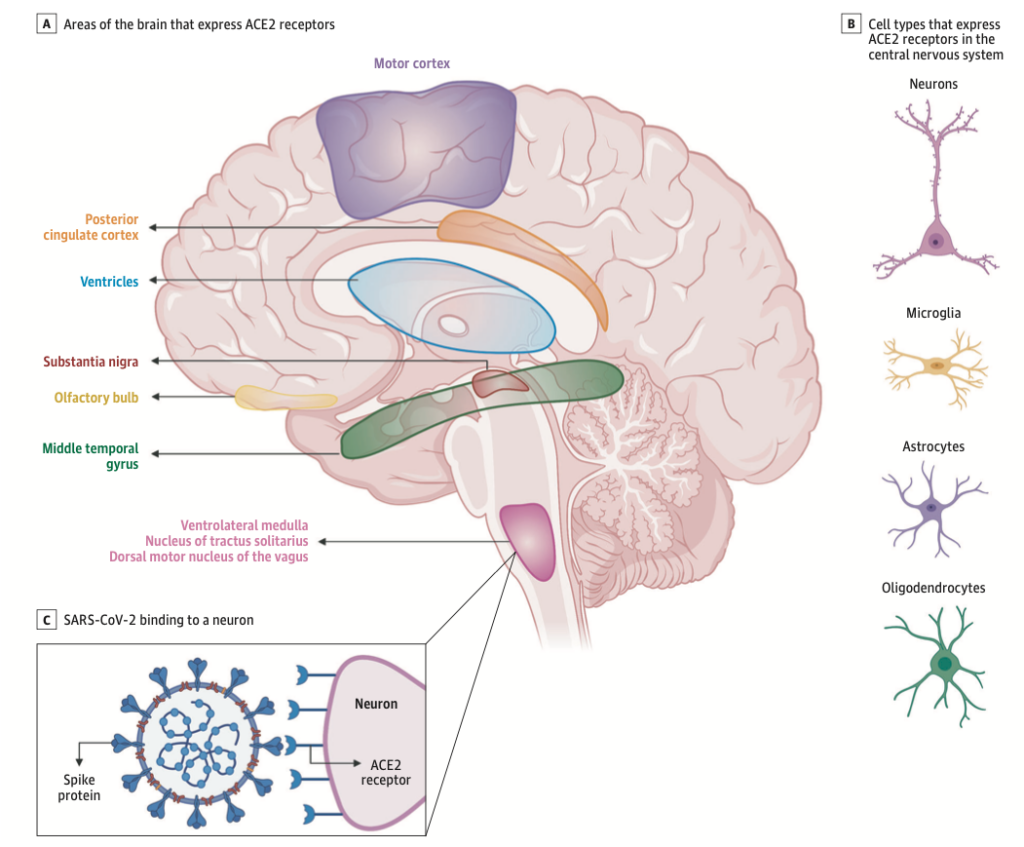
Viral Infections and Autoimmune Disorders: Predictive value of Non-specific Symptoms such as Hyposmia
There is growing evidence of neurological complications and disease in patients with COVID-19. Two similar human coronaviruses (CoV), Middle East respiratory syndrome (MERS-CoV) and severe acute respiratory syndrome (SARS-CoV-1), have also been associated with neurological disease in rare cases (Lechien et al., 2020). This raises the questions of whether SARS-CoV-2 is neurotropic and whether it contributes to post-infectious neurologic complications. A handful of case reports have described neurological complications in patients with COVID-19 (Zubair et al., 2020).
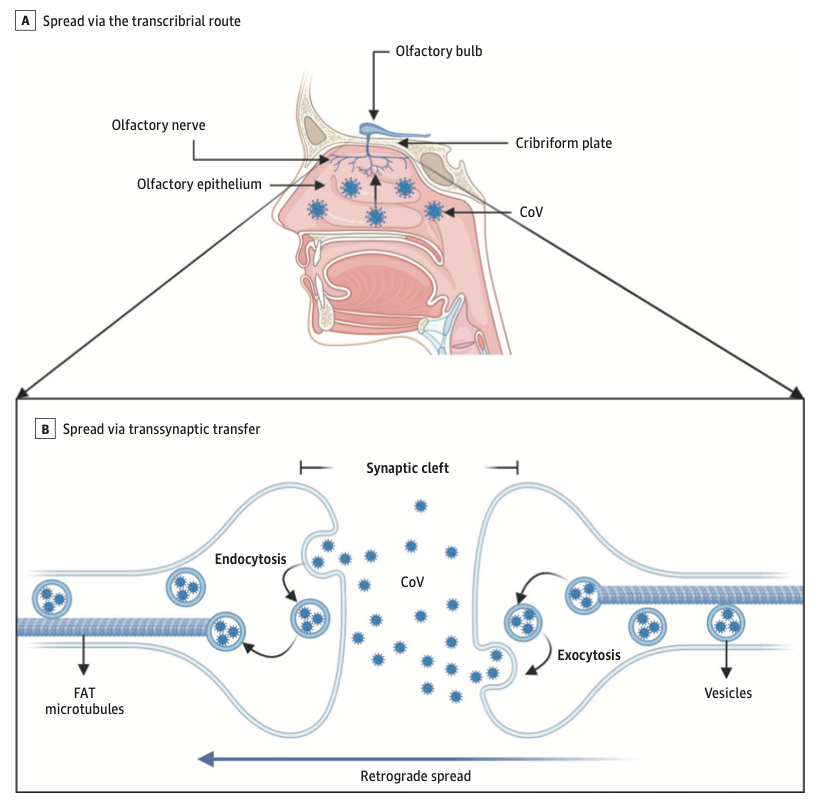
The prevalence of anosmia and ageusia ranges widely in the literature. In a study of patients hospitalized in Wuhan, the prevalence of hypogeusia was 5.6% and 5.1% respectively, while 19.4% of patients in Italy had some form of chemosensory dysfunction (Lechien et al., 2020). Approximately 88.5% and 88.0% of patients in Germany reported olfactory and gustatory dysfunction respectively. Of patients without nasal congestion, 79.7% were hyposmic. Anosmia has also been noted in other respiratory infections, such as influenza. In COVID-19 anosmia is typically not accompanied by nasal swelling or rhinitis, casting suspicion on the possible nasal passage inflammation, and maybe indicative of neurological dysfunctions in the central olfactory pathways (Caggiu et al., 2019). Although non-specific, given the reports of anosmia and/or hyposmia presenting as an early symptom in many other neurological disorders such as PD, MS, and Guillain-Barré after viral infections such as the flu, one cannot help but to correlate viral causes with some neurological disorders with autoimmune origins (Leger et al., 2020; Olsen et al., 2018; Qiao et al., 2019). As described in Figure 7 Infected vascular endothelial cells have been shown to spread severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to glial cells in the central nervous system. Known as the Trojan horse mechanism, infected leukocytes can cross the blood-brain barrier to infect the central nervous system (Chen et al., 2020; Lechien et al., 2020; Qiao et al., 2019; Zubair et al., 2020).
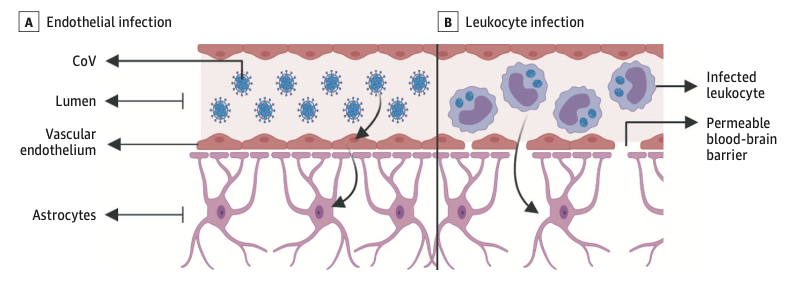
Putting the Pieces Together
As provided above, in the past few decades, abundance of literature has emerged hailing viruses as the possible origin of many autoimmune disorders. From herpes to viruses causing seasonal flu, neurological disorders are common epiphenomena emerging with these infectious processes (Caggiu et al., 2019). As explained earlier, the intriguing fact remains the ability of these viral pathogens to enter cells, change their genetic makeup, and above all cause damage to neurons in the central nervous system. Although easier said than done, this receptor-mediated target-specific destructive force creates an amazing possibility to predict and deliver genetic changes to neurons in specific regions of the brain. Imagine targeting foci causing epilepsy, mitotic changes in neoplasm, or even changing apoptotic processes in aging, neurodegenerative diseases and stroke (Gonzalez-Alegre, 2007). Not to compare this to the moon landing, but Jules Verne’s “From the Earth to the Moon” predicted an impossible journey that came true a few decades later. It is only a matter of time before we decode the mysterious world of viruses and using them as biological vesicles for re-programming native cells to cure disease and hopefully not for evil purposes. Perhaps the time has finally come to move on from optogenetics and embark on virogenetics.
References
Alcohol Metabolism—An overview | ScienceDirect Topics. (n.d.). Retrieved May 31, 2020, from https://www.sciencedirect.com/topics/medicine-and-dentistry/alcohol-metabolism
Allan, C. (2007). Awakenings. BMJ : British Medical Journal, 334(7604), 1169. https://doi.org/10.1136/bmj.39227.715370.59
Andersen, B. B. (2004). Reduction of Purkinje cell volume in cerebellum of alcoholics. Brain Research, 1007(1–2), 10–18. https://doi.org/10.1016/j.brainres.2004.01.058
Baker, K. G., Harding, A. J., Halliday, G. M., Kril, J. J., & Harper, C. G. (1999). Neuronal loss in functional zones of the cerebellum of chronic alcoholics with and without Wernicke’s encephalopathy. Neuroscience, 91(2), 429–438. https://doi.org/10.1016/s0306-4522(98)90664-9
Barbeau, A., Roy, M., Bernier, G., Campanella, G., & Paris, S. (1987). Ecogenetics of Parkinson’s disease: Prevalence and environmental aspects in rural areas. The Canadian Journal of Neurological Sciences. Le Journal Canadien Des Sciences Neurologiques, 14(1), 36–41. https://doi.org/10.1017/s0317167100026147
Bodiga: Renin angiotensin system in cognitive function… – Google Scholar. (n.d.). Retrieved June 1, 2020, from
Caggiu, E., Arru, G., Hosseini, S., Niegowska, M., Sechi, G., Zarbo, I. R., & Sechi, L. A. (2019). Inflammation, Infectious Triggers, and Parkinson’s Disease. Frontiers in Neurology, 10. https://doi.org/10.3389/fneur.2019.00122
Chen, R., Wang, K., Yu, J., Chen, Z., Wen, C., & Xu, Z. (2020). The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in human and mouse brain. BioRxiv, 2020.04.07.030650. https://doi.org/10.1101/2020.04.07.030650
Dale, R. C., Church, A. J., Surtees, R. A. H., Lees, A. J., Adcock, J. E., Harding, B., Neville, B. G. R., & Giovannoni, G. (2004). Encephalitis lethargica syndrome: 20 new cases and evidence of basal ganglia autoimmunity. Brain: A Journal of Neurology, 127(Pt 1), 21–33. https://doi.org/10.1093/brain/awh008
D’Ardes, D., Boccatonda, A., Rossi, I., Guagnano, M. T., Santilli, F., Cipollone, F., & Bucci, M. (2020). COVID-19 and RAS: Unravelling an Unclear Relationship. International Journal of Molecular Sciences, 21(8), 3003. https://doi.org/10.3390/ijms21083003
Dong, X. (2018). Current Strategies for Brain Drug Delivery. Theranostics, 8(6), 1481–1493. https://doi.org/10.7150/thno.21254
Fahn, S. (1996). Book Review. New England Journal of Medicine, 335(26), 2002–2003. https://doi.org/10.1056/NEJM199612263352618
Gonzalez-Alegre, P. (2007). Monomelic parkinsonian tremor caused by contralateral substantia nigra stroke. Parkinsonism & Related Disorders, 13(3), 182–184. https://doi.org/10.1016/j.parkreldis.2006.03.011
Grammatopoulos, T. N., Jones, S. M., Ahmadi, F. A., Hoover, B. R., Snell, L. D., Skoch, J., Jhaveri, V. V., Poczobutt, A. M., Weyhenmeyer, J. A., & Zawada, W. M. (2007). Angiotensin type 1 receptor antagonist losartan, reduces MPTP-induced degeneration of dopaminergic neurons in substantia nigra. Molecular Neurodegeneration, 2, 1. https://doi.org/10.1186/1750-1326-2-1
Hamming, I., Timens, W., Bulthuis, M. L. C., Lely, A. T., Navis, G. J., & van Goor, H. (2004). Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology, 203(2), 631–637. https://doi.org/10.1002/path.1570
Jackson, L., Eldahshan, W., Fagan, S. C., & Ergul, A. (2018). Within the Brain: The Renin Angiotensin System. International Journal of Molecular Sciences, 19(3). https://doi.org/10.3390/ijms19030876
Jang, H., Boltz, D. A., Webster, R. G., & Smeyne, R. J. (2009). Viral Parkinsonism. Biochimica et Biophysica Acta, 1792(7), 714–721. https://doi.org/10.1016/j.bbadis.2008.08.001
Kelly, H. (n.d.). WHO | The classical definition of a pandemic is not elusive. WHO; World Health Organization. Retrieved June 2, 2020, from https://www.who.int/bulletin/volumes/89/7/11-088815/en/
Langston, J. W. (2017). The MPTP Story. Journal of Parkinson’s Disease, 7(Suppl 1), S11–S19. https://doi.org/10.3233/JPD-179006
Lechien, J. R., Chiesa-Estomba, C. M., De Siati, D. R., Horoi, M., Le Bon, S. D., Rodriguez, A., Dequanter, D., Blecic, S., El Afia, F., Distinguin, L., Chekkoury-Idrissi, Y., Hans, S., Delgado, I. L., Calvo-Henriquez, C., Lavigne, P., Falanga, C., Barillari, M. R., Cammaroto, G., Khalife, M., … Saussez, S. (2020). Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. European Archives of Oto-Rhino-Laryngology: Official Journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): Affiliated with the German Society for Oto-Rhino-Laryngology – Head and Neck Surgery. https://doi.org/10.1007/s00405-020-05965-1
Leger, C., Herbert, M., & DeSouza, J. F. X. (2020). Non-motor Clinical and Biomarker Predictors Enable High Cross-Validated Accuracy Detection of Early PD but Lesser Cross-Validated Accuracy Detection of Scans Without Evidence of Dopaminergic Deficit. Frontiers in Neurology, 11. https://doi.org/10.3389/fneur.2020.00364
Lenkei, Z., Palkovits, M., Corvol, P., & Llorens-Cortès, C. (1997). Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: A functional neuroanatomical review. Frontiers in Neuroendocrinology, 18(4), 383–439. https://doi.org/10.1006/frne.1997.0155
Mao, L., Jin, H., Wang, M., Hu, Y., Chen, S., He, Q., Chang, J., Hong, C., Zhou, Y., Wang, D., Miao, X., Li, Y., & Hu, B. (2020). Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurology. https://doi.org/10.1001/jamaneurol.2020.1127
Neuropathology of Alcohol-Related Brain Damage | Alcohol and Alcoholism | Oxford Academic. (n.d.). Retrieved May 31, 2020, from https://academic.oup.com/alcalc/article/44/2/136/184817
Olsen, L. K., Dowd, E., & McKernan, D. P. (2018). A role for viral infections in Parkinson’s etiology? Neuronal Signaling, 2(2). https://doi.org/10.1042/NS20170166
Qiao, X.-F., Wang, G.-P., Li, X., Bai, Y.-H., & Zheng, W. (2019). Analysis of the clinical effect of olfactory training on olfactory dysfunction after upper respiratory tract infection. Acta Oto-Laryngologica, 139(7), 643–646. https://doi.org/10.1080/00016489.2019.1614224
Role of H1N1 Influenza Virus in the Etiology of Parkinson’s Disease. (n.d.). The Michael J. Fox Foundation for Parkinson’s Research | Parkinson’s Disease. Retrieved May 31, 2020, from https://www.michaeljfox.org/grant/role-h1n1-influenza-virus-etiology-parkinsons-disease
Sacks, O. (1983). The origin of “Awakenings”. British Medical Journal (Clinical Research Ed.), 287(6409), 1968–1969.
Snow, B. J., Vingerhoets, F. J. G., Langston, J. W., Tetrud, J. W., Sossi, V., & Calne, D. B. (2000). Pattern of dopaminergic loss in the striatum of humans with MPTP induced parkinsonism. Journal of Neurology, Neurosurgery & Psychiatry, 68(3), 313–316. https://doi.org/10.1136/jnnp.68.3.313
Sullivan, E. V., Rosenbloom, M. J., Deshmukh, A., Desmond, J. E., & Pfefferbaum, A. (1995). Alcohol and the Cerebellum. Alcohol Health and Research World, 19(2), 138–141.
The Effect of Ethanol on Human Brain Metabolites Longitudinally Characterized by Proton MR Spectroscopy—Armin Biller, Andreas J Bartsch, György Homola, Làszló Solymosi, Martin Bendszus, 2009. (n.d.). Retrieved May 31, 2020, from https://journals.sagepub.com/doi/full/10.1038/jcbfm.2009.12
Vaduganathan, M., Vardeny, O., Michel, T., McMurray, J. J. V., Pfeffer, M. A., & Solomon, S. D. (2020). Renin–Angiotensin–Aldosterone System Inhibitors in Patients with Covid-19. New England Journal of Medicine, 382(17), 1653–1659. https://doi.org/10.1056/NEJMsr2005760
Vlot, A. H. C., de Witte, W. E. A., Danhof, M., van der Graaf, P. H., van Westen, G. J. P., & de Lange, E. C. M. (2017). Target and Tissue Selectivity Prediction by Integrated Mechanistic Pharmacokinetic-Target Binding and Quantitative Structure Activity Modeling. The AAPS Journal, 20(1), 11. https://doi.org/10.1208/s12248-017-0172-7
WHO | What is a pandemic? (n.d.). WHO; World Health Organization. Retrieved June 2, 2020, from http://www.who.int/csr/disease/swineflu/frequently_asked_questions/pandemic/en/
Wright, J. W., & Harding, J. W. (2013). The brain renin-angiotensin system: A diversity of functions and implications for CNS diseases. Pflugers Archiv: European Journal of Physiology, 465(1), 133–151. https://doi.org/10.1007/s00424-012-1102-2
Xia, H., & Lazartigues, E. (2008). Angiotensin‐converting enzyme 2 in the brain: Properties and future directions. Journal of Neurochemistry, 107(6), 1482–1494. https://doi.org/10.1111/j.1471-4159.2008.05723.x
Zubair, A. S., McAlpine, L. S., Gardin, T., Farhadian, S., Kuruvilla, D. E., & Spudich, S. (2020). Neuropathogenesis and Neurologic Manifestations of the Coronaviruses in the Age of Coronavirus Disease 2019: A Review. JAMA Neurology. https://doi.org/10.1001/jamaneurol.2020.2065
Remy Cohan
Dr.Remy Cohan MD,PhD