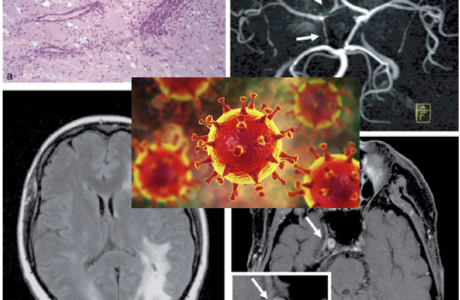
Abstract
Lupus anticoagulant (LA) is associated clinically with thrombosis, not bleeding; most patients with LA do not have systemic lupus erythematosus. The laboratory hallmark of LA is a prolonged aPTT that does not correct when normal plasma is added, which in turn indicates the presence of an inhibitor of clotting as opposed to a deficiency of a necessary coagulation factor. Here, we look at the evidence behind the role of antiphospholipid antibodies (APLAs) in data available from patients with antiphospholipid antibody syndrome (APS) and the role of immunoglobulins in thrombosis .
- 1- Antiphospholipid antibodies are immunoglobulins
- 2- APS syndrome and recurrent ischemic stroke: APS in pregnancy vs. catastrophic APS
- 3- Conclusion
- 4- References
1- Antiphospholipid antibodies are immunoglobulins
Antiphospholipid antibodies (APAs) are usually IgG or IgM antibodies that bind to negatively charged phospholipids (Alving et al., 1990). Phospholipids are important constituents of vascular endothelium, heart proteins, platelets, and other cells (Hanly, 2003). Laboratory studies show that sera from patients with APLAs are anticardiolipin antibody and the so-called lupus anticoagulant (LA). LAs are acquired immunoglobulins that are associated clinically with thrombosis (Fleetwood et al., 2018). Most patients with LA do not have systemic lupus (Panichpisal et al., 2012). The laboratory hallmark of LA is a prolonged aPTT that does not correct when normal plasma is added. This indicates that deficiency is coagulation factor is not the underlying cause but the presence of an inhibitor of clotting (Fleetwood et al., 2018).
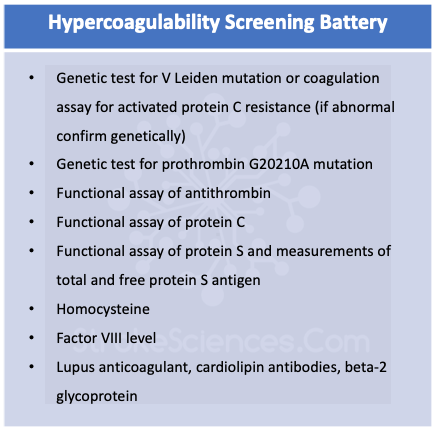
LA can be detected using a sensitive phospholipid reagent, the kaolin clotting time, or the russel viper venom time. Anticardioli[in antibodies of the IgG or IgM types can be measured as well as more specific antibodies to beta-2-glycoprotein1 (Panichpisal et al., 2012). IgG antibody levels, especially those greater than 40 GPL (IgG phospholipid) units, corelate with a relatively high risk of stroke and recurrent stroke. Antiphospholipid antibodies are actually antibodies to a protein, most often beta-2-glycoprotein 1, that is usually bound to a phospholipid. Measurement of beta-2-glycoprotein 1 antibodies is often useful in defining autoimmune activity. Two other antibodies, antiphosphatidyl serine and antiphosphatylinositol, show a high correlation with ischemic stroke especially in the young and in those without another determined cause (Fleetwood et al., 2018; Hanly, 2003; Schreiber et al., 2018). The screening tests for syphilis- the VDRL and Reiter protein reaction- depend on the activity of APLAs. False-positive serological tests for syphilis are often found in patients with APAs, and thrombocytopenia os present in a third of APA-postive patients (Fleetwood et al., 2018).
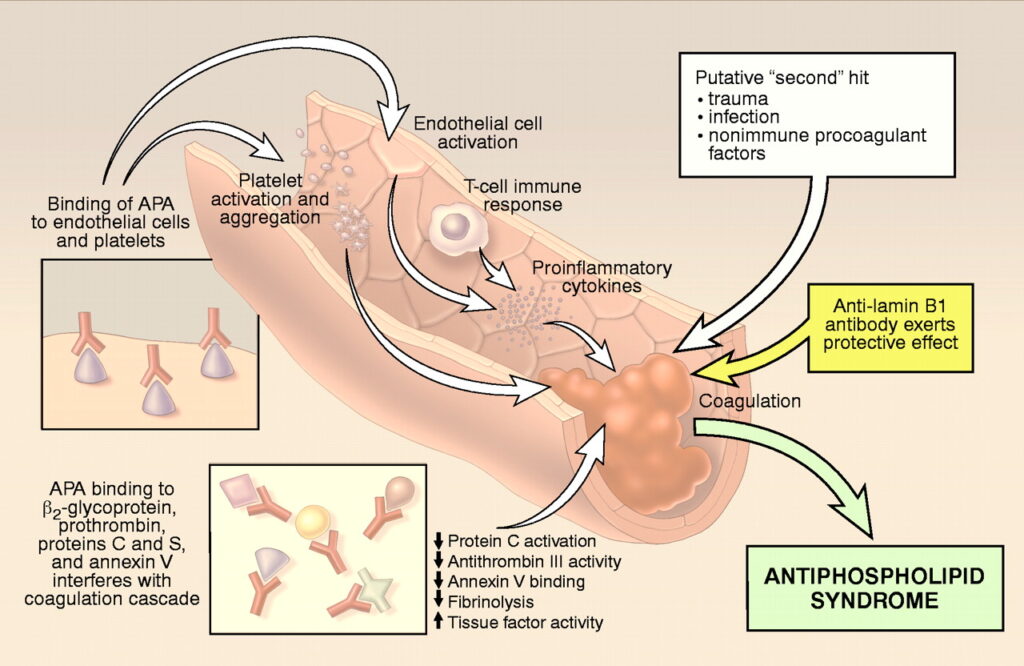
The clinical APLA syndrome consists of frequent venous and arterial thrombotic events, such as thrombophlebitis, pulmonary embolism, TIAs, myocardial infarctions, and recurrent fetal loss in women. It is likely that the mechanism of recurrent brain ischemia is excessive clotting. APLAs may be direct ed against the endothelium or platelet membranes and may alter coagulability and vascular functions. APLAs should be measured in situations in which the usual risk factors for ischemic stroke are not present, and certainly in patients with livedo reticularis and strokes (Sneddon’s syndrome), and those patients with clinical features matching the primary APS (Alving et al., 1990; Fleetwood et al., 2018).
2- APS syndrome and recurrent ischemic stroke: APS in pregnancy vs. catastrophic APS
APS consists of thrombosis and (in pregnancy) fetal demise caused by various antibodies directed against one or more phospholipid-bound proteins (eg, beta- 2glycoprotein 1, prothrombin, annexin A5). Annexin A5 may bind to phospholipid membrane constituents to prevent the cell membrane from initiating the activation of coagulation. If autoantibodies displace annexin A5, procoagulant endothelial cell surfaces may be exposed and provoke arterial or venous thromboses. The precise mechanism of thrombosis in patients with autoantibodies to phospholipid-bound beta2 glycoprotein 1 is unknown. In a small proportion of patients with APS, widespread thromboses occur in small vessels supplying multiple organs, often causing neurologic defects (Alving et al., 1990; Fleetwood et al., 2018). This syndrome is called catastrophic APS (CAPS) and can be confused with disseminated intravascular coagulation (DIC), heparin-induced thrombocytopenia (HIT), and thrombotic microangiopathy (TMA) (Schreiber et al., 2018). Its treatment includes high-dose corticosteroids, anticoagulation, plasmapheresis, and sometimes rituximab, an anti-CD20 monoclonal antibody, or eculizumab, an anti-complement component 5 (C5) monoclonal antibody (Fleetwood et al., 2018).
3- Conclusion
APS despite its rare occurrence in stroke population provides another window into the role of immunoglobulins in intravascular pathologies and thrombus formation. Reports of thromboembolic events after systemic infections are ever increasing occurrences that have changed the work up required in patients with infectious related coagulability and/or genetic risk factors. Especially when it comes to the unorthodox stroke presentations in the younger population with no known risk factors.
4- References
Alving, B. M., Barr, C. F., & Tang, D. B. (1990). Correlation between lupus anticoagulants and anticardiolipin antibodies in patients with prolonged activated partial thromboplastin times. The American Journal of Medicine, 88(2), 112–116. https://doi.org/10.1016/0002-9343(90)90458-p
Antiphospholipid Antibody Syndrome | National Heart, Lung, and Blood Institute (NHLBI). (n.d.). Retrieved May 17, 2020, from https://www.nhlbi.nih.gov/health-topics/antiphospholipid-antibody-syndrome
Fleetwood, T., Cantello, R., & Comi, C. (2018). Antiphospholipid Syndrome and the Neurologist: From Pathogenesis to Therapy. Frontiers in Neurology, 9. https://doi.org/10.3389/fneur.2018.01001
Hanly, J. G. (2003). Antiphospholipid syndrome: An overview. CMAJ, 168(13), 1675–1682. Moore, G. W. (2016). Current Controversies in Lupus Anticoagulant Detection. Antibodies, 5(4). https://doi.org/10.3390/antib5040022
Schreiber, K., Sciascia, S., de Groot, P. G., Devreese, K., Jacobsen, S., Ruiz-Irastorza, G., Salmon, J. E., Shoenfeld, Y., Shovman, O., & Hunt, B. J. (2018). Antiphospholipid syndrome. Nature Reviews Disease Primers, 4(1), 1–20. https://doi.org/10.1038/nrdp.2017.103
Panichpisal, K., Rozner, E., & Levine, S. R. (2012). The management of stroke in antiphospholipid syndrome. Current Rheumatology Reports, 14(1), 99–106. https://doi.org/10.1007/s11926-011-0223-5
Remy Cohan
Dr. Remy Cohan MD, PhD