Abstract
Although infection-related stroke is not a new topic, recent SARS-CoV-2 or COVID-19 pandemic has caused a widespread media coverage on this topic. Claims and Reports of cardiovascular and neurological symptoms in infected populations have sparked an ever increasing interest in understanding the underlying mechanisms of thromboembolic events in the presence of systemic infections. Here, a brief review of literature and facts are provided, in order to understand these mechanisms better.
- 1- Case Reports and Research models linking infection to thromboembolic events
- 2- Vasculitis and other possibly inflammatory vascular disorders
- 3- Hypercoagulable state due to infection and the disseminated intravascular coagulation
- 4- Conclusion
- 5- References
1- Case Reports and Research models linking infection to thromboembolic events
In the last weeks of April 2020, a Milanese group, Lodigiani and colleagues (2020), from one of the epicentres of COVID-19, published a paper reporting thromboembolic events in a cohort of 388 patients admitted to the univeristy hospital of Milan. They conclude: “The high number of arterial and, in particular, venous thromboembolic events diagnosed within 24 h of admission and the high rate of positive VTE imaging tests among the few COVID-19 patients tested suggest that there is an urgent need to improve specific VTE diagnostic strategies and investigate the efficacy and safety of thromboprophylaxis in ambulatory COVID-19 patients“. Prior to this study, Song et al., (2020), a Chinese group, also reported that “Studies coming out of Wuhan have shown that coagulation dysfunction is a major cause of death in patients with severe COVID-19”
These reports and a cascade of other reports mainly from China and Spain started the quest for understanding the link between the SARS-CoV-2 infection and thromboembolic events including multiple reports of stroke. In general anything that causes abnormalities of the clotting system can lead to hypercoagulability and thrombosis. Even in patients with lesions known to predispose to thromboembolism and brain ischemia, the acute event is often precipitated by a change n the blood and its coagulability. Infections, inflammatory bowel disease, and cancer are examples of conditions that are associated with release of acute-phase reactants that may alter coagulability sufficiently to promote thromboembolism-especially if there is a preexisting lesion that affects an endothelial surface (Moghtaderi & Alavi-Naini, 2012). Bleeding diatheses often cause intracranial bleeding. Abnormalities of the viscosity of blood can alter blood flow, especially in small arterioles and capillaries of the brain, and in patients with blood occlusive lesions. Increased viscosity can cause or contribute to regional decreases in cerebral blood flow (CBF) and potentiate ischemia. Autoimmunity, which can be detected and monitored by blood test, can lead to occlusive cerebrovascular disease (Jillella & Wisco, 2019). Case reports, reporting acute upper respiratory infection and stroke like symptoms both in adults and the paediatric populations have long been published and discussed. For example, Zilkha and colleagues in 1976, described:
“Three children presented with acute onset of hemiplegia following an upper respiratory infection. Angiography revealed irregularities, beading, and slow flow of a peripheral branch of a middle cerebral artery. In addition, one child had narrowing of the cervical segment of the internal carotid artery. An inflammatory arteritis of the cervical internal carotid artery is presumably the site of the formation of cerebral emboli.“
In animal models, a model of acute respiratory distress syndrome (ARDS), mice infected intranasally with a lethal dose of influenza virus were shown to have increased platelet aggregation, pulmonary microvascular thrombosis, endothelial damage and hyper-inflammatory cytokine responses (Beristain-Covarrubias et al., 2019).
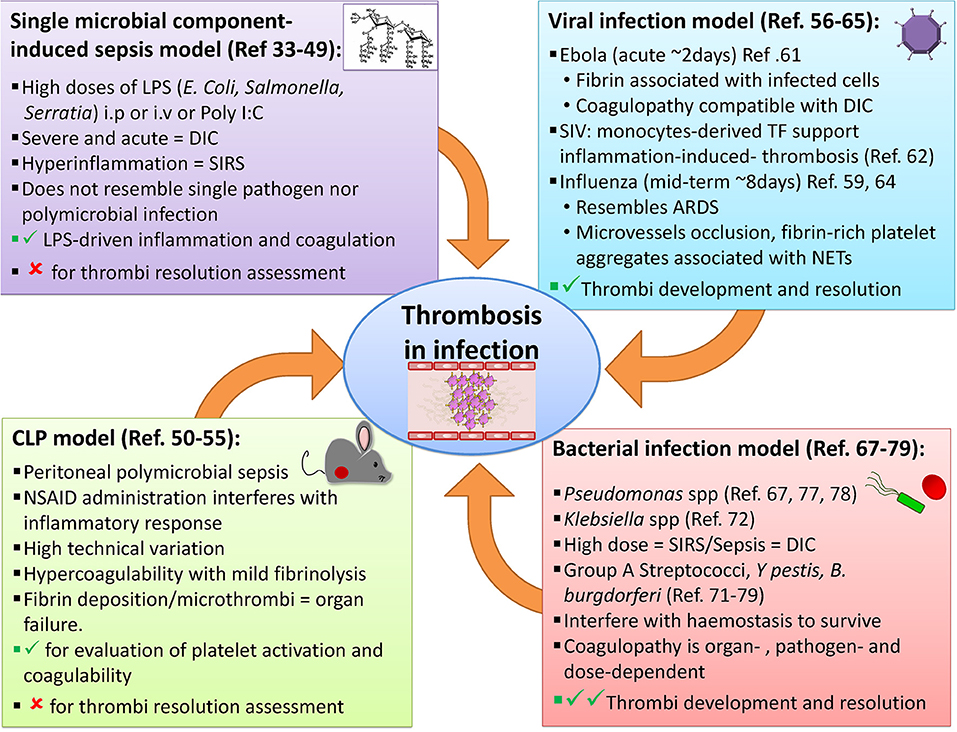
These accounts clearly describe, a cascade of thromboembolic events that is potentially caused by infectious agents and/or is caused as an epiphenomenon of an overworked immune system. According to these reports and knowledge already existed, two important mechanisms lead to such thromboembolic events following an infection:
1- Inflammation in arterial walls and damage to the endothelial cells and the vascular smooth muscle cells (potentially causing thrombus)
2- Hypercoagulable state due to infection (disseminated intravascular coagulation and other causes)
2- Vasculitis and other possibly inflammatory vascular disorders
Arteritis (angiits) is one of the causes of stroke in the differential diognosis of nearly every medical student and non-neurologists. Although, often considered, documented arteritis is a rare cause of stroke (Russo et al., 1995; Schmidt et al., 2016). Most often, central nervous system (CNS) vasculitis presents as an encephalopathy with headache, seizure, decreased alertness, and cognitive and behavioural abnormalities, often with multifocal signs. Recognition of those rare instances in which arteritis is caused by specific microbial infection is critical for effective treatment. Patients with allergic hypersensitivity and systemic vasculitis may respond to corticosteriods or other treatments used to control systemic autoimmune diseases (Schmidt et al., 2016).
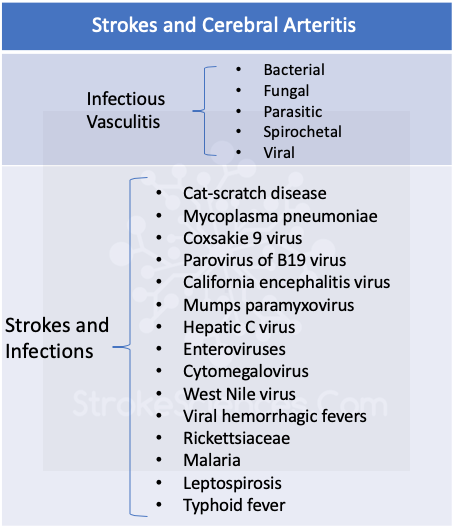
3- Hypercoagulable state due to infection and the disseminated intravascular coagulation
Sepsis is frequently associated with disseminated intravascular coagulation (DIC), a critical presentation of altered blood coagulation and microthrombus formation in the microvascular bed of different organs (Beristain-Covarrubias et al., 2019). The risk of thrombotic complications after infection is not limited to the hospital setting. There is clear evidence that in the community setting, infections increase the risk of venous thromboembolic complications (DVT/PE), with the host and the pathogen both determining the outcome of this relationship (Beristain-Covarrubias et al., 2019; Hermida et al., 1998). In SIRS and DIC, inflammation is mediated by multiple cytokines such as interleukins 1, 6, and 8 (IL-1,−6, and−8), interferons (IFNs) and tumor necrosis factor α (TNFα) (Nagel et al., 2010). Moreover, there is a strong association with damage-associated molecular pattern (DAMPs) molecules like DNA and histones, both as free molecules and within neutrophil extracellular traps (NETs), which are released by activated leucocytes and also promote thrombi formation (Hermida et al., 1998). These combine to promote the pro-coagulant state leading to endothelial damage, platelet activation and aggregation, increases in pro-coagulant proteins such as tissue factor (TF), and reduced activity of anticoagulant mechanisms like fibrinolysis. Compounding this, pathogens themselves are often capable of modulating inflammation and the coagulation system through the production of either pro- or anti-coagulant proteins (Hermida et al., 1998).
Conclusion
Although we are dealing with a new strain of coronavirus (SARS-CoV-2), it is important to realize that changes in concentration of blood products, inflammatory markers, as well as an over-worked immune system, have been known to cause thromboembolic events. This knowledge predates SARS-CoV-2, and as a matter of fact, in last few decades usage of anticoagulants such as heparin has been a standard of care in treating carefully selected patients with DIC.
5- References
Beristain-Covarrubias, N., Perez-Toledo, M., Thomas, M. R., Henderson, I. R., Watson, S. P., & Cunningham, A. F. (2019). Understanding Infection-Induced Thrombosis: Lessons Learned From Animal Models. Frontiers in Immunology, 10. https://doi.org/10.3389/fimmu.2019.02569
Frontiers | Understanding Infection-Induced Thrombosis: Lessons Learned From Animal Models | Immunology. (n.d.). Retrieved May 16, 2020, from https://www.frontiersin.org/articles/10.3389/fimmu.2019.02569/full
Goldberg, M. F., Goldberg, M. F., Cerejo, R., & Tayal, A. H. (2020). Cerebrovascular Disease in COVID-19. American Journal of Neuroradiology. https://doi.org/10.3174/ajnr.A6588
Hermida, J., Montes, R., Páramo, J. A., & Rocha, E. (1998). Endotoxin-induced disseminated intravascular coagulation in rabbits: Effect of recombinant hirudin on hemostatic parameters, fibrin deposits, and mortality. The Journal of Laboratory and Clinical Medicine, 131(1), 77–83. https://doi.org/10.1016/s0022-2143(98)90080-4 http://fyra.io. (n.d.).
Infectious Causes of Stroke. Practical Neurology; Bryn Mawr Communications. Retrieved May 16, 2020, from https://practicalneurology.com/articles/2020-jan/infectious-causes-of-stroke
Jillella, D. V., & Wisco, D. R. (2019). Infectious causes of stroke. Current Opinion in Infectious Diseases, 32(3), 285–292. https://doi.org/10.1097/QCO.0000000000000547
Lodigiani, C., Iapichino, G., Carenzo, L., Cecconi, M., Ferrazzi, P., Sebastian, T., Kucher, N., Studt, J.-D., Sacco, C., Alexia, B., Sandri, M. T., & Barco, S. (2020). Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thrombosis Research, 191, 9–14. https://doi.org/10.1016/j.thromres.2020.04.024
Massberg, S., Grahl, L., von Bruehl, M.-L., Manukyan, D., Pfeiler, S., Goosmann, C., Brinkmann, V., Lorenz, M., Bidzhekov, K., Khandagale, A. B., Konrad, I., Kennerknecht, E., Reges, K., Holdenrieder, S., Braun, S., Reinhardt, C., Spannagl, M., Preissner, K. T., & Engelmann, B. (2010). Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nature Medicine, 16(8), 887–896. https://doi.org/10.1038/nm.2184
Miller, E. C., & Elkind, M. S. V. (2016). Infection and Stroke: An Update on Recent Progress. Current Neurology and Neuroscience Reports, 16(1), 2. https://doi.org/10.1007/s11910-015-0602-9
Moghtaderi, A., & Alavi-Naini, R. (2012). Infective Causes of Stroke in Tropical Regions. Iranian Journal of Medical Sciences, 37(3), 150–158. Nagel, M. A., Mahalingam, R., Cohrs, R. J., & Gilden, D. (2010). Virus Vasculopathy and Stroke: An Under-Recognized Cause and Treatment Target. Infectious Disorders Drug Targets, 10(2), 105–111.
Russo, M. G., Waxman, J., Abdoh, A. A., & Serebro, L. H. (1995). Correlation between infection and the onset of the giant cell (temporal) arteritis syndrome. A trigger mechanism? Arthritis and Rheumatism, 38(3), 374–380. https://doi.org/10.1002/art.1780380312
Schmidt, J., Smail, A., Roche, B., Gay, P., Salle, V., Pellet, H., & Duhaut, P. (2016). Incidence of Severe Infections and Infection-Related Mortality During the Course of Giant Cell Arteritis: A Multicenter, Prospective, Double-Cohort Study. Arthritis & Rheumatology (Hoboken, N.J.), 68(6), 1477–1482. https://doi.org/10.1002/art.39596
Senst, B., Tadi, P., Basit, H., & Jan, A. (2020). Hypercoagulability. In StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK538251/
Song, J.-C., Wang, G., Zhang, W., Zhang, Y., Li, W.-Q., Zhou, Z., & People’s Liberation Army Professional Committee of Critical Care Medicine, C. S. on T. and H. (2020). Chinese expert consensus on diagnosis and treatment of coagulation dysfunction in COVID-19. Military Medical Research, 7(1), 19. https://doi.org/10.1186/s40779-020-00247-7
Zilkha, A., Mendelsohn, F., & Borofsky, L. G. (1976). Acute hemiplegia in children complicating upper respiratory infections. Report of three cases with angiographic findings. Clinical Pediatrics, 15(12), 1137–1142. https://doi.org/10.1177/000992287601501209
Remy Cohan
Dr.Remy Cohan MD, PhD