NMES
Neuromuscular Electrical Stimulation (NMES) offers a promising intervention for post-stroke rehabilitation by directly stimulating motor neurons, inducing muscle contractions, and promoting neuroplasticity. In stroke survivors with hemiparesis or plegia, particularly those affected by damage to the motor pathways, NMES has been found to play a pivotal role in reactivating weakened or paralyzed muscles and facilitating the brain’s adaptive reorganization. By understanding the physiological processes underlying muscle contraction, motor pathway damage, and neuroplastic potential, we can maximize the benefits of NMES for patients recovering from stroke.
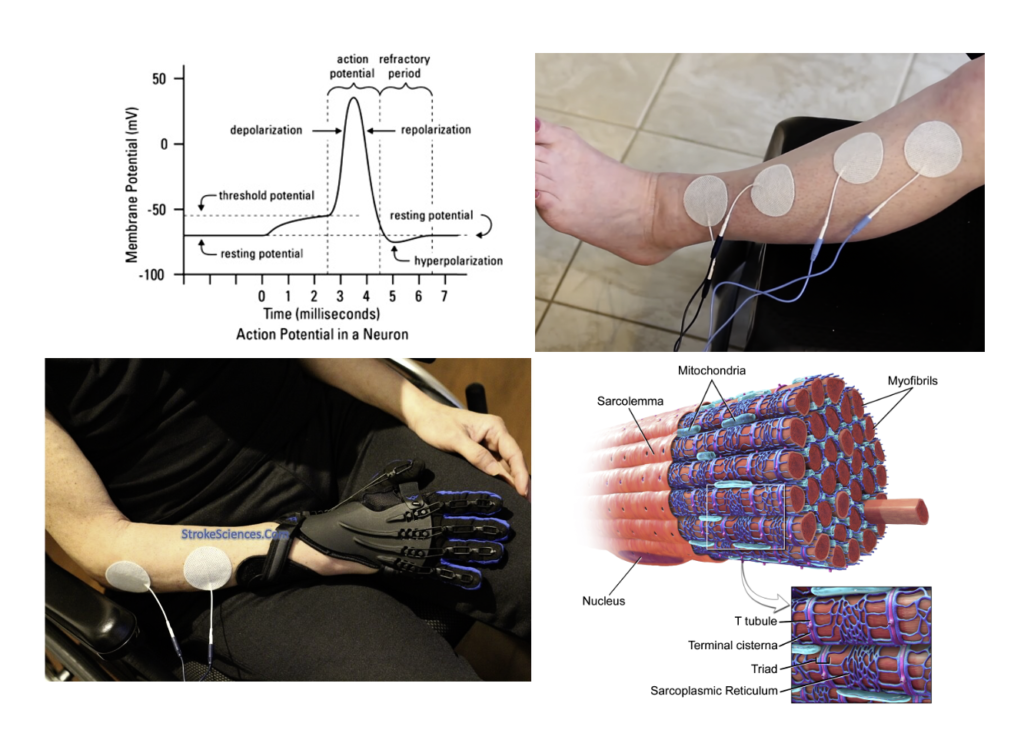
Muscle contraction is governed by motor units, which consist of a single motor neuron and the muscle fibres it innervates. Each muscle fibre is composed of sarcomeres, the fundamental units responsible for contraction. Sarcomeres contain two primary protein filaments: actin, anchored at the Z-line, and myosin, anchored at the M-line. When stimulated, the myosin heads attach to the actin filaments, pulling them inward toward the M-line in a process known as the sliding filament model. This action shortens the sarcomere and, consequently, the muscle fibre, generating contraction. Activation of these muscle fibres occurs at the neuromuscular junction, where the motor neuron releases the neurotransmitter acetylcholine upon receiving an action potential. Acetylcholine binds to receptors on the muscle membrane, leading to membrane depolarization, calcium ion release, and initiation of the contractile process.
Action Potentials, Graded Potentials, and NMES Application
NMES bypasses the typical neural pathway by directly stimulating the motor neurons through electrical currents, creating an artificial electric field that induces a graded potential in the neuron. If the graded potential reaches the threshold, it initiates an action potential, which propagates along the neuron to the muscle fibres, replicating voluntary contraction. While an action potential is an all-or-nothing response, a graded potential varies in strength, depending on the NMES current’s intensity, amplitude, and pulse width. By adjusting these parameters, NMES can control the threshold level for action potential generation, allowing graded potentials to summate and create sustained muscle contractions, a critical element for therapeutic muscle strengthening and re-education.
Electrode Placement and the Role of Electric Field Polarity in NMES
In devices like the Neurotech AvivaStim XP, biphasic waveforms are used, meaning the electric current alternates between positive and negative polarities in each cycle. This alternating current ensures that both electrodes periodically serve as the active electrode, so the orientation of positive and negative electrodes does not impact the effectiveness of NMES. The generated electric field stimulates the motor neurons regardless of electrode polarity, as the only requirement is that the field reaches the threshold needed to create a sufficient graded potential. Proper electrode placement, however, remains essential, especially for dorsiflexion in stroke patients (watch the video). For instance, the tibialis anterior can be effectively targeted by placing one electrode on the muscle’s motor point (located one-third down from the knee) and the second electrode on the muscle belly, just above the ankle. This configuration optimizes the electric field’s distribution, maximizing the stimulation effect on the muscle.
Hemiparesis and Hemiplegia: Motor Pathway Damage Following Stroke
Stroke is one of the primary causes of hemiparesis (weakness on one side of the body) and hemiplegia (paralysis on one side of the body), with the location of the stroke significantly influencing the motor deficits observed. In cases where a stroke occurs in the motor cortex, patients may experience more localized motor impairments due to the highly organized nature of the motor homunculus, where specific cortical regions control distinct body parts. Damage to the motor cortex results in deficits corresponding to the specific area of the body that the affected cortical region controls. For example, a stroke impacting the motor cortex region associated with leg function may lead to weakness or paralysis in the leg, while the rest of the body remains unaffected.
However, strokes affecting subcortical structures, brainstem or the internal capsule often lead to more profound motor impairments due to their role as a bottleneck for descending motor fibres. The internal capsule is a compact, V-shaped structure containing axons that connect the cerebral cortex with other brain regions and the spinal cord. This anatomical structure is responsible for carrying motor signals from the motor cortex to lower motor neurons in the spinal cord. Consequently, damage to these regions disrupt a vast number of motor pathways, leading to more extensive hemiparesis or hemiplegia that can affect the entire side of the body.
NMES as a Tool for Neuroplasticity and Motor Recovery
The potential for NMES to enhance recovery lies in its ability to promote neuroplasticity, a process through which the brain reorganizes itself to form new connections, compensating for damaged motor pathways. By repeatedly stimulating the paralyzed or weakened muscle fibres, NMES sends continuous feedback signals to the brain, facilitating synaptic strengthening in motor pathways. Over time, this repetitive stimulation can lead to reorganization within the motor cortex, allowing undamaged areas of the brain to assume control over the affected muscles. This process is especially crucial for patients with hemiparesis or hemiplegia, as it offers a pathway to restore lost functions even when primary motor pathways are compromised.
NMES Settings and Their Therapeutic Applications
The effectiveness of NMES in generating muscle contractions and inducing neuroplasticity depends heavily on specific device settings, including frequency, amplitude, and pulse width. Each parameter influences the nature of the muscle contraction and, consequently, the therapeutic outcome.
Frequency, measured in Hertz (Hz), represents the number of pulses per second. Lower frequencies, such as those between 1 and 10 Hz, produce slow, twitch-like contractions, beneficial for initial muscle activation in cases of severe atrophy or early rehabilitation. Medium frequencies (20-35 Hz) produce more controlled, smooth contractions, which are particularly useful for managing spasticity in stroke patients. Higher frequencies, around 50 Hz or more, induce tetanic contractions, where the muscle remains fully contracted throughout the stimulation period. These higher frequencies are ideal for muscle strengthening and re-education, as they maximize motor unit recruitment.
Amplitude, measured in milliamps (mA), controls the intensity of the electric current, with higher amplitudes generating stronger electric fields. This increased field strength enhances the likelihood of reaching the graded potential threshold needed to initiate an action potential. Low amplitude is used for sensitive or initial rehabilitation phases, while moderate to high amplitudes are applied when more substantial muscle contractions are needed, such as in strength-building or large muscle group stimulation.
Pulse width, measured in microseconds (us), defines the duration of each electrical pulse. Short pulse widths (80-200 us) provide brief stimuli, which are less likely to reach the action potential threshold, making them suitable for delicate or minimally invasive muscle activation. Longer pulse widths (300-400 us) allow for stronger stimulation, effective for activating larger muscles or for patients with reduced muscle excitability due to paralysis or weakness.
Therapeutic Applications of the Neurotech AvivaStim XP Programs
The Neurotech AvivaStim XP device offers various NMES programs tailored for different therapeutic needs. These programs vary in frequency, pulse width, contraction-relaxation cycles, and intended treatment time. For instance, Program 1 operates at 50 Hz with a 5-second contraction and 5-second relaxation period, making it ideal for basic muscle re-education in early rehabilitation phases. Program 3, with a 50 Hz frequency, 10-second contraction, and 20-second relaxation, is suited for strength building and motor re-education in post-stroke recovery, especially for patients requiring longer contraction times to build endurance.
Programs with lower frequencies, such as Program 5 (10 Hz with 5-second cycles), are effective for gentle muscle reactivation and early rehabilitation in severely weakened muscles. Conversely, programs with dual-frequency channels, such as Program 6 (Channel 1 at 50 Hz and Channel 2 at 10 Hz), allow for coordinated dual-muscle activation, beneficial in complex motor tasks like gait training.
| Program No. | Frequency (Hz) | Contraction (sec.) | Relaxation (sec.) | Ramp Up (sec.) | Ramp Down (sec.) | Pulse Width (us) | Burst/Trigger | Treatment Time (mins) |
|---|---|---|---|---|---|---|---|---|
| 1 | 50 | 5 | 5 | 1 | 1 | 300 | Trigger | 30 |
| 2 | 50 | 5 | 10 | 1 | 1 | 300 | Trigger | 30 |
| 3 | 50 | 10 | 20 | 1.5 | 1.5 | 400 | Trigger | 30 |
| 4 | 35 | 5 | 5 | 1 | 1 | 300 | Trigger | 30 |
| 5 | 10 | 5 | 5 | 0.5 | 0.5 | 300 | Trigger | 30 |
| 6 | Channel 1: 50, Channel 2: 10 | 5 | 5 | 0.5 | 0.5 | 300 | None | 30 |
| 7 | 35 | 5 | 5 | 1 | 0.5 | 350 | None | 30 |
| 8 | 8 | 5 | 5 | 1 | 0.5 | 80 | Trigger | 30 |
| 9 | 4-99 | Continuous | None | 0.5 | 0.5 | 150 | Trigger | Open |
Bottomline
NMES stands out as a powerful tool in stroke rehabilitation, offering targeted stimulation to re-engage motor pathways and muscle fibres affected by hemiparesis or hemiplegia. By understanding the physiological basis of muscle contraction and the motor pathway disruptions caused by stroke, clinicians can apply NMES effectively to facilitate neuroplasticity and recovery. In a selected patient population, through repeated stimulation, NMES not only aids in muscle strengthening but also enhances the brain’s adaptive capacity. In some cases, this retraining can successfully elicit neuroplastic changes, helping stroke patients regain functional control and independence over their movements.
You can watch the NMES videos here: Ankle dorsiflexion, Wrist & digit flexion