Abstract
It is thought that over 50% of the population do not have a complete circle of Willis. This important web of arteries is a vital pathway for collateral blood flow to protect against ischemia. Racial and gene-related congenital morphologic variants could be one of the main reasons why vascular blockage leads to a variety of signs and symptoms and severity. This article utilizes historical evidence and modern epidemiological literature to answer the above question.
- Introduction
- Genetic variation and congenital anatomical differences
- Racial Disparities in Stroke, Angiogenesis and Arteriogenesis
- Moyamoya Disease
- Conclusion
- References
Introduction
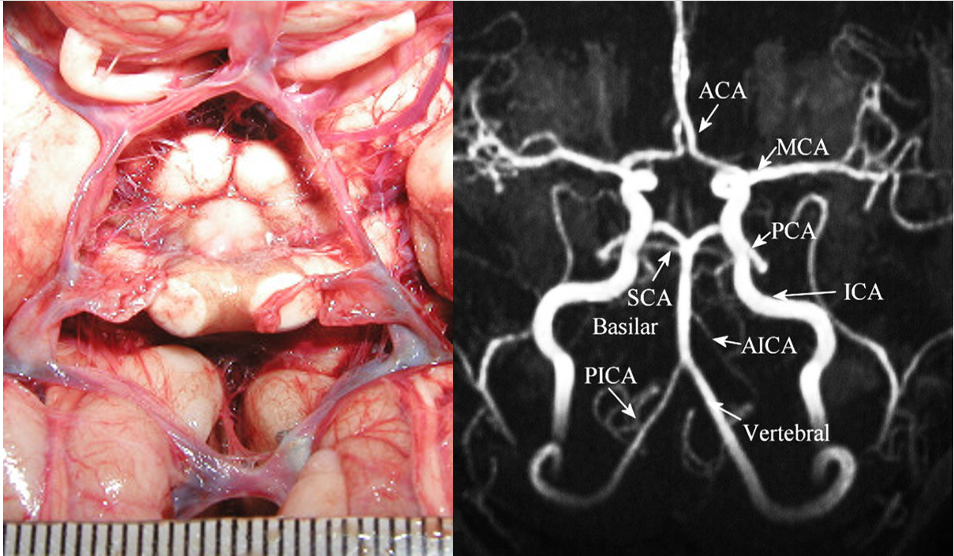
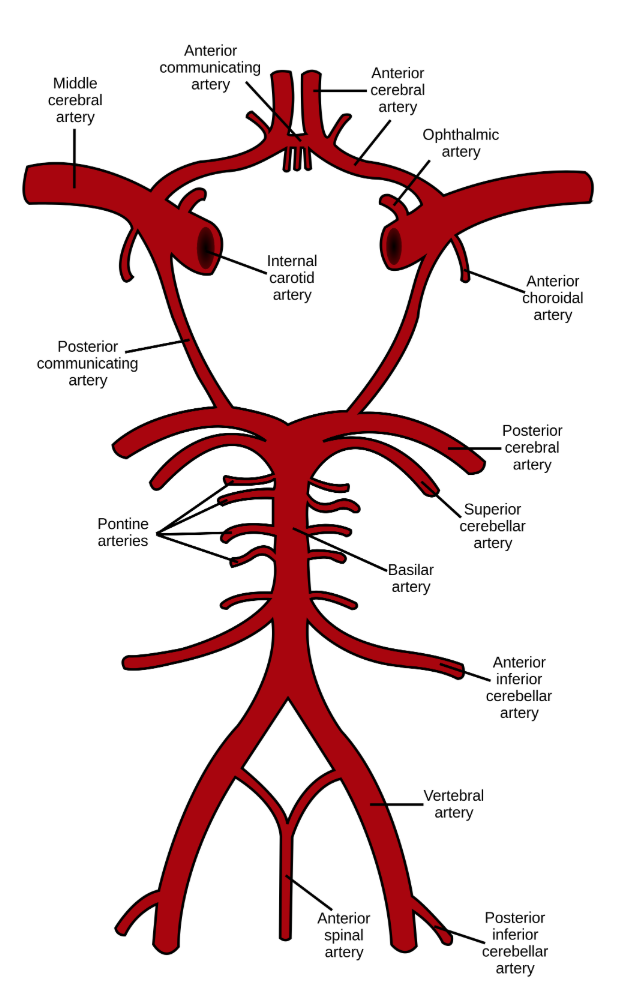
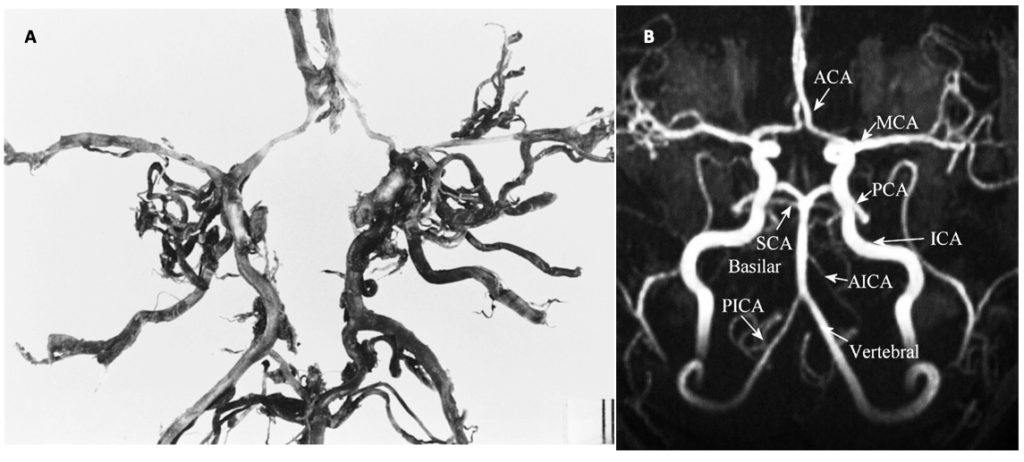
The main arterial blood supply to the brain is through the left and the right internal carotid arteries and the vertebrobasilar system. Anastomoses between these vessels form the circle of Willis (CoW) (Figure 1). We have known about this web of arteries at the base of the brain since the 16th century, after mentions of it in a work done by Gabriel Fallopius (1523-1562), a century later, descriptions of the circle and its anastomotic function was furnished by John Jakob Wepfer (1658), and as history goes, the victor is Thomas Willis (1621–1675) who contributed the term “neurology” to medicine, and was the first person to describe the clinical importance of this circle (Lo & Ellis, 2010).
Later on, Richard Lower (1631-1691) an associate of WIllis, demonstrated that the sufficiency of the arterial circle at the base of the brain is required to maintain the cerebral circulation, even when three of the four arteries supplying the brain had been tied off. In 1839, Ellis reported the astonishing experience of successful ligation of both carotid arteries in a 21 year old patient who sustained a gunshot wound of the neck and tongue when he was mistaken for a bear by a companion. On October 21, 1844, approximately a week later the incident, Ellis had to ligate the patient’s left carotid artery because of hemorrhage from the tongue wound. There was a recurrent hemorrhage on the eleventh day after the accident, and they soon realized that application of pressure on the right carotid artery helped control the blood loss. Therefore, it was necessary to ligate the right carotid artery as well, only four and a half days after the left carotid artery had been ligated (Catlin, 1960). Ellis remarked:
“For convenience we had him in the sitting position during the operation; when we tightened the ligature, no disagreeable effects followed; no fainting, no bad feeling about the head; and all perceptible change was a slight paleness, a cessation of pulsation in both temporal arteries, and of the hemorrhage.”
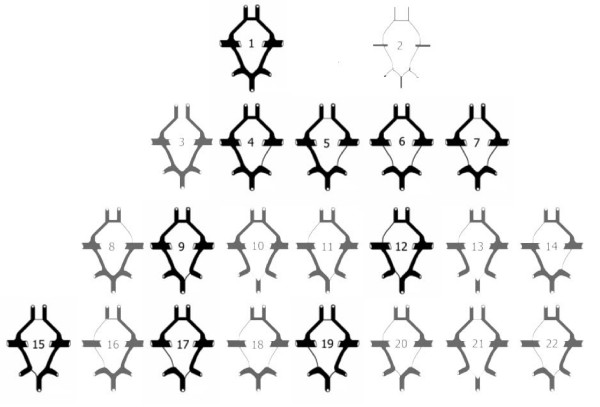
Although this would not have been possible, had the unfortunate patient not had a complete CoW, these historical events all point at the lucky 50% with a gene pool that provides a complete CoW and adequate collateral circulation. We now know, that multiple genetic and environmental factors contribute to the formation, variation and modification of the circle and the collateral blood flow (Lo & Ellis, 2010). These factors become very important in the presence of cerebral vascular pathologies and can play a detrimental role in outcome. Although not always, a complete CoW has been attributed to better outcomes after cerebral ischemia (Lazorthes et al., 1979). In the following sections genetic variations and morphological difference will be explored in order to better understand the underlying differences in different populations.
Genetic variation and congenital anatomical differences
Deformities in the CoW can significantly increase the risk of cerebrovascular disease in humans. However, the molecular mechanisms underlying these deformities have not been understood. Animal Studies show that variations in the CoW are hereditary (Harel et al., 2015; Li et al., 2015). A normal CoW is observed in approximately 60% of mouse models, a percentage that also applies to humans (Lazorthes et al., 1979). To study the mechanisms underlying these variations, researchers selected genes associated with different types of the CoW. After evaluating genetic differences, 4 genes: CST3, GNAS, GPx4 and PFN2 were associated with variations in the CoW (Harel et al., 2015; Li et al., 2015). We may assume that these 4 genes play an important role in the development of variations in the CoW. Such studies provide a foundation for further research of genes related to development of variations in the CoW and the mechanisms of dysmorphosis of cerebral vessels (Li et al., 2015).
Racial Disparities in Stroke, Angiogenesis and Arteriogenesis
Before delving deeper into the anatomical and congenital variabilities of the CoW, it is important to talk about angiogenesis and arteriogenesis. Arteriogenesis, also known as collateral growth, is an adaptive response to transient, repetitive coronary or cerebral artery occlusion such as occurs in stable angina pectoris, carotid and vertebral artery occlusions (Rafat Neysan et al., 2009) and has been associated with lower incidence and severity of stroke and myocardial infarction. It has been shown that collateral development is impaired in patients suffering from type II diabetes and the metabolic syndrome (Ooi et al., 2016). Remodelling of a preexisting collateral arteriole is thought to be caused by flow-induced changes secondary to occlusion of a supply artery. The consequent increase in shear stress through the collateral arteriole activates endothelial cells, resulting in monocyte recruitment and infiltration into the media. Elaboration of various cytokines, growth factors, and proteases from monocytes and endothelial cells causes matrix degradation, smooth muscle cell proliferation, and rapid enlargement of the preexisting arteriole (Saito & Imai, 2011). Factors thought to promote arteriogenesis include FGF-2, placental growth factor, PDGF-BB, TGFβ1, monocyte chemoattractant protein 1, and GM-CSF.
In contrast to angiogenesis, defined as de novo vessel (capillary tube) formation, arteriogenesis is defined by enlargement of small arterioles (i.e., capillaries), with very low or no blood flow, to larger conducting arteries (Ooi et al., 2016). This process begins with endothelial activation, increased expression of VCAM-1 and ICAM-1 and increased endothelial permeability, all of which allow for adhesion of inflammatory cells, mostly monocytes. Next VSMCs switch to the synthetic and proliferative phenotype and migrate across the internal elastic lamina and the basement membrane into the lumen of the preexisting vessel in an inward remodeling process akin to neointima formation following vascular injury (Mukawa et al., 2016). This is followed by reestablishment of the internal elastic lamina and the basement membrane as well as the endothelium, and outward remodeling in which cells migrate across the external elastic lamina into the adventitia and the surrounding myocardium, thus allowing for vessel expansion and significant increases in blood flow. The genetic downside of carrying variants in RNF213 lead to susceptibility to moyamoya disease. Certain alleles of RNF213 lead to a Mendelian disease of early onset, whereas other alleles confer susceptibility to moyamoya disease (Nishijima et al., 2015). Such a paradigm is apparent for many neurodegenerative diseases (e.g., MAPT in autosomal dominant frontotemporal dementia, sporadic progressive supranuclear palsy, and late-onset Parkinson disease (Antonarakis et al., 2010); Martin et al., 2001]) and for disorders of lipid metabolism (e.g., LDLR variants in familial hypercholesterolemia and sporadic disease (Kathiresan et al., 2009; Varret et al., 2008). This perhaps can be seen to complement another situation wherein a homozygous mutation gives rise to a monogenic Mendelian disorder while a heterozygous carrier state leads to susceptibility to a multifactorial disease, as evident for Gaucher disease and susceptibility to Parkinson disease (Sidransky et al., 2009), cystic fibrosis and susceptibility to pancreatic insufficiency (Cohn et al., 1998; Sharer et al., 1998), Stargardt disease and susceptibility to age-related macular degeneration (Shroyer et al., 1999; Allikmets et al., 1997), and others (Mukawa et al., 2016).
Moyamoya Disease
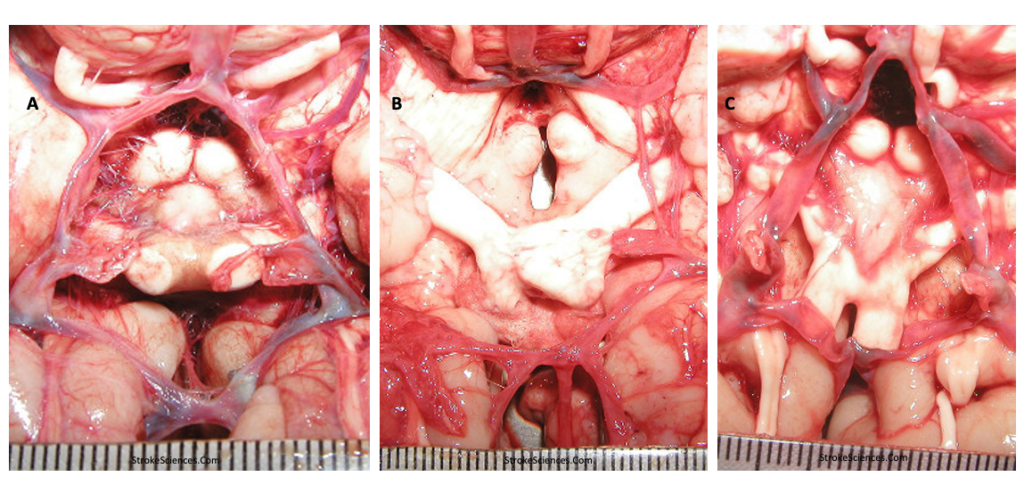
Although arteriogenesis or angiogenesis are processes that are generally helpful in dodging ischemic processes, they can cause moyamoya, a disease affecting the cerebral arteries, which predisposes individuals to recurrent ischemic attacks in association with progressive steno-occlusive change of the intracranial internal carotid arteries and their proximal branches (De Silva et al., 2011; Lazorthes et al., 1979; Nishijima et al., 2015). The disorder is typically characterized by a reduction in blood flow in the major vessels of the anterior circulation of the brain. This results in the development of a compensatory collateral vasculature around the stenosed vessels, near the apex of the internal carotid, on the cortical surface, leptomeninges, and branches of the external carotid artery and the base of the skull (Saito & Imai, 2011). In advanced cases, the posterior circulation is also involved, including the basilar and posterior cerebral arteries. While it was first described as a “hypoplasia of the bilateral internal carotid arteries,” the characteristic appearance of the network of abnormally dilated collateral vessels on conventional angiography was compared to “a puff of cigarette smoke” (Arch Neurol 20(3):288–899, 1969) or “moyamoya” in Japanese. Moyamoya disease patients are predominantly found in Eastern Asian countries such as Japan and the Republic of Korea, although recent studies epidemiological studies in the US also suggest that Hispanics and African-Americans also at a greater risk for moyamoya disease, but available evidence suggests that these populations are mainly predisposed to moyamoya disease secondary due to high blood pressure and diabetic propensities, and not due to genetic variations involved in the disease. Moyamoya disease patients show bimodal distribution, adult type and pediatric type. Symptom of adult moyamoya patients can be either ischemic or hemorrhagic; nevertheless, pediatric patients usually present with ischemia (Iqbal, 2013; Saito & Imai, 2011).
Conclusion
Given the above descriptions, and the vital role of genetic predisposition in congenital vascular deformities, it is evident that a syndrome such as stroke is a multifaceted pathology. Although such genetic variabilities and racial disparities do not change outcome In the acute stroke settings, patient education and lifestyle modifications in populations with higher incidence of congenital vascular disease can dramatically decrease the burden of disability and all cause mortality associated with stroke. This may be an important point in patient education, and clinical consideration in stroke prevention consults both for patients and their offsprings.
References
Catlin, D. (1960). A Case of Carcinoma of the Larynx Surviving Bilateral Carotid Artery Ligation. Annals of Surgery, 152(5), 809–814.
De Silva, K. R. D., Silva, R., Amaratunga, D., Gunasekera, W., & Jayesekera, R. W. (2011). Types of the cerebral arterial circle (circle of Willis) in a Sri Lankan Population. BMC Neurology, 11, 5. https://doi.org/10.1186/1471-2377-11-5
Harel, T., Posey, J. E., Graham, B. H., Walkiewicz, M., Yang, Y., Lalani, S. R., & Belmont, J. W. (2015). Atypical Presentation of Moyamoya Disease in an Infant with a de novo RNF213 Variant. American Journal of Medical Genetics. Part A, 167(11), 2742–2747. https://doi.org/10.1002/ajmg.a.37230
Iqbal, S. (2013). A Comprehensive Study of the Anatomical Variations of the Circle of Willis in Adult Human Brains. Journal of Clinical and Diagnostic Research : JCDR, 7(11), 2423–2427. https://doi.org/10.7860/JCDR/2013/6580.3563
Lazorthes, G., Gouazé, A., Santini, J.-J., & Salamon, G. (1979). Le cercle artériel du cerveau (circulus arteriosus cerebri). Anatomia clinica, 1(3), 241–257. https://doi.org/10.1007/BF01654581
Li, Z., Huo, X., Zhang, S., Lu, J., Li, C., Guo, M., Fu, R., He, Z., Du, X., & Chen, Z. (2015). Selection of Genes Associated with Variations in the Circle of Willis in Gerbils Using Suppression Subtractive Hybridization. PLOS ONE, 10(5), e0127355. https://doi.org/10.1371/journal.pone.0127355
Lo, W. B., & Ellis, H. (2010). The circle before willis: A historical account of the intracranial anastomosis. Neurosurgery, 66(1), 7–18; discussion 17-18. https://doi.org/10.1227/01.NEU.0000362002.63241.A5
Mukawa, M., Nariai, T., Inaji, M., Tamada, N., Maehara, T., Matsushima, Y., Ohno, K., Negi, M., & Kobayashi, D. (2016). First autopsy analysis of a neovascularized arterial network induced by indirect bypass surgery for moyamoya disease: Case report. Journal of Neurosurgery, 124(5), 1211–1214. https://doi.org/10.3171/2015.4.JNS15155
Nishijima, Y., Akamatsu, Y., Weinstein, P. R., & Liu, J. (2015). Collaterals: Implications in cerebral ischemic diseases and therapeutic interventions. Brain Research, 1623, 18–29. https://doi.org/10.1016/j.brainres.2015.03.006
Ooi, Y. C., Laiwalla, A. N., Liou, R., & Gonzalez, N. R. (2016). Angiographic Structural Differentiation between Native Arteriogenesis and Therapeutic Synangiosis in Intracranial Arterial Steno-Occlusive Disease. American Journal of Neuroradiology, 37(6), 1086–1091. https://doi.org/10.3174/ajnr.A4675
Rafat Neysan, Beck Grietje Ch., Peña-Tapia Pablo G., Schmiedek Peter, & Vajkoczy Peter. (2009). Increased Levels of Circulating Endothelial Progenitor Cells in Patients With Moyamoya Disease. Stroke, 40(2), 432–438. https://doi.org/10.1161/STROKEAHA.108.529420
Saito, N., & Imai, H. (2011). Insights on the revascularization mechanism for treatment of Moyamoya disease based on the histopathologic concept of angiogenesis and arteriogenesis. World Neurosurgery, 75(2), 204–205. https://doi.org/10.1016/j.wneu.2010.10.004
. Remy Cohan
Dr. Remy Cohan MD,PhD